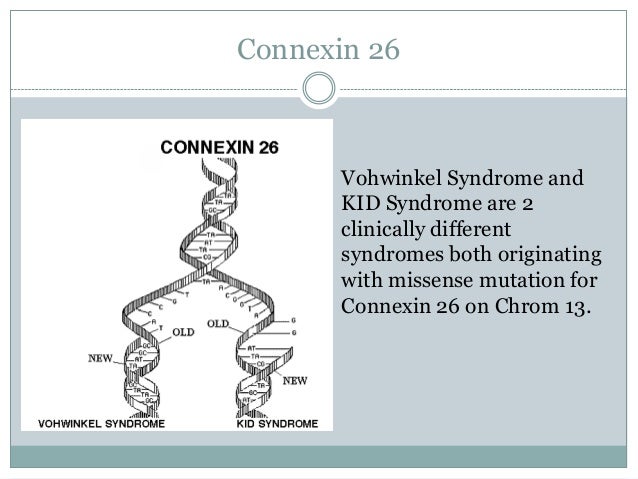
Vohwinkel syndrome
vohwinkel syndrome
is a disorder with classic and variant forms, both of which affect the skin.
The classic form of
Vohwinkel syndrome is caused by mutations in the GJB2 gene. This gene provides instructions
for making a protein called gap junction beta 2, more commonly known as
connexin 26. Connexin 26 is a member of the connexin protein family. Connexin
proteins form channels called gap junctions that permit the transport of
nutrients, charged atoms (ions), and signaling molecules between neighboring
cells that are in contact with each other. Gap junctions made with connexin 26
transport potassium ions and certain small molecules.
Connexin 26 is found in cells throughout the
body, including the inner ear and the skin. In the inner ear, channels made
from connexin 26 are found in a snail-shaped structure called the cochlea.
These channels may help to maintain the proper level of potassium ions required
for the conversion of sound waves to electrical nerve impulses. This conversion
is essential for normal hearing. In addition, connexin 26 may be involved in
the maturation of certain cells in the cochlea. Connexin 26 also plays a role
in the growth, maturation, and stability of the outermost layer of skin (the
epidermis).
The GJB2 gene mutations that cause Vohwinkel
syndrome change single protein building blocks (amino acids) in connexin 26.
The altered protein probably disrupts the function of normal connexin 26 in
cells, and may interfere with the function of other connexin proteins. This
disruption could affect skin growth and also impair hearing by disturbing the
conversion of sound waves to nerve impulses.